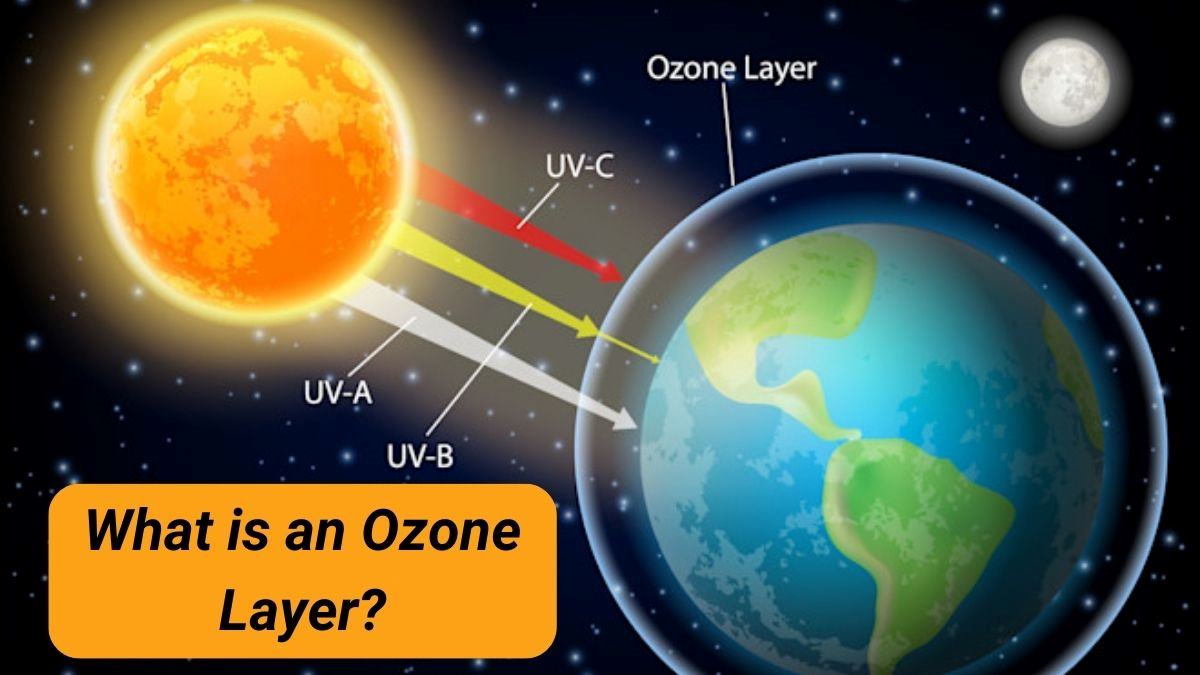
What is the ozone layer?
The ozone layer is a key component of the Earth’s stratosphere, located between 19 and 30 km above the surface. It plays a crucial role in filtering out harmful ultraviolet (UV) radiation from the sun, thus protecting life on Earth.
- Optical Illusion Test Your Vision: Can You Find The Hidden Metal Chain Within 10 Seconds?
- Optical Illusion Brain Test: If you have Sharp Eyes Find the number 1419 among 1914 in 7 Seconds?
- Can You Find a Key in this Store Room in 12 Seconds? Explanation and Solution to the Optical Illusion
- Donald Trump Withdraws U.S. from Paris Climate Accord in Second Term: What It Means?
- Optical Illusion Brain Test: Oh No! The Rabbit Is Missing In The Hat. Do You See Where Is It Hiding?
.jpg)
Source: Ourworldindata/Hannah Ritchie
Function of the ozone layer
- Absorb UV radiation: Prevent excessive UV-B and UV-C rays from reaching the Earth.
- Protect organisms: Reduce risks of weakened immune systems such as skin cancer, cataracts and weak immune systems.
- Conservation of ecosystems: Shield marine life, especially plankton, is crucial to the marine food chain.
- Prevent material degradation: Protect paints, fabrics and other materials from UV damage.
Why is the ozone layer important?
The protective shielded UV ozone layer serves as the earth’s sunscreen, absorbing 97-99% of harmful ultraviolet (UV-B) radiation. Without it, all living things will be exposed to excessive UV light, causing DNA damage, skin cancer, environmental damage, and affecting marine ecosystems and plant life.
Why is the southern hemisphere affected by ozone depletion?

Source: GML.NOAA
Studies have shown that ozone depletion is more obvious in the southern hemisphere, especially in high latitude areas. This is due to the presence of cooler temperatures, which contribute to the formation of polar stratospheric clouds. These clouds accelerate the chemical reactions that break down ozone molecules. In contrast, ozone depletion is rarely near the equator and deteriorates towards the pole, highlighting the role of temperature and sunlight in this phenomenon.
Why do ozone pores form?
- Polar vortex: Style captures cold air on the Antarctic to prevent rich ozone air from mixing.
- Stratospheric cloud: Extreme cold can lead to cloud formation, enhancing the ozone depletion response.
- Spring Activation: When sunlight returns from September to October, chemical reactions accelerate, causing a sharp drop in ozone levels.
- Self-healing mechanism: In summer, new ozone flows in from lower latitudes, temporarily closing the hole.
Effects of ozone depletion on ultraviolet radiation
UV radiation is divided into three types:
|
UV type |
Wavelength (NM) |
Effect |
|
UV-A |
315-400 nm |
The least harmful can lead to aging and wrinkles. |
|
UV-B |
280-315 nm |
Causes skin cancer, cataracts and immunosuppression. |
|
UV-C |
<280 nm |
Highly dangerous, but mainly absorbed by the ozone layer. |
Even a small amount of ozone reduction can significantly increase UV-B radiation, thereby increasing risks to human health and ecosystems.
Ozone layer depletion and its connection to climate change
Although ozone depletion and climate change are separate phenomena, they have some interconnected aspects.
|
Ozone depletion |
Climate change |
|
Caused by chlorofluoro compounds (CFCs) and other ozone animals (ODS). |
Mainly powered by greenhouse gases (GHG) such as carbon dioxide and methane. |
|
Causes an increase in ultraviolet radiation on the Earth’s surface. |
Capture heat, leading to global warming. |
|
Causes health problems, crop damage and substance degradation. |
Change weather patterns to melt the ice sheet and increase sea level. |
|
While CFC is prohibited, it still exists in the atmosphere. |
Carbon dioxide emissions continue to increase. |
Causes of ozone depletion

Source: GML.NOAA
- Chlorofluoro compounds (CFCS): found in refrigerants, aerosols and industrial solvents. They release chlorine atoms that destroy ozone molecules.
- Halon and bromine compounds: used in fire extinguishers and pesticides.
- Nitrous oxide (N2O): emitted by combustion of fertilizers and fossil fuels, resulting in ozone degradation.
Ozone destruction mechanism
- CFC decomposes in the stratosphere due to ultraviolet radiation.
- Release chlorine atoms and then react with ozone (O3) molecules.
- A chlorine atom can destroy up to 100,000 ozone molecules.
- This process depletes the concentration of ozone, allowing more ultraviolet radiation to penetrate the Earth’s surface.
What causes ozone depletion?
The crisis from the 1970s to the 1980s
Human activities release large quantities of ozone animals (ODS), such as CFC. Ozone levels in the stratosphere decreased significantly. Ozone pores appeared, allowing more UV-B radiation to reach the earth’s surface.
Global Success Story: Ozone Recovery
Montreal Agreement (1987) and Global Action Results:
- Gradually eliminate substances of ozone.
- The ozone layer is gradually recovering.
- Millions of skin cancer cases have been prevented.
One of the biggest international environmental success stories!
Milestones for ozone protection
- 1977: GML begins monitoring CFC and ozone animals.
- 1987: The Montreal Agreement was adopted and the production of CFC was phased out.
- 1996: Industrialized countries prohibit the use of CFC.
- 2020s: Ozone pores show signs of recovery, but monitoring continues.

Source: OurWorldIndata
Ozone monitoring and global efforts
The Global Monitoring Laboratory (GML) part of NOAA plays a key role in ozone observation.
Ozone monitoring method
- DOBSON spectrophotometer: Measures total ozone levels at 16 global sites.
- Ozonesondes: A weather balloon equipped with sensors tracks the vertical profile of ozone.
- Satellite Observation: Verify ground measurements and track global ozone trends.
- Halogenation analysis: Monitoring chlorine and bromine compounds that cause ozone loss.
in conclusion
The ozone layer remains the key shield to protecting life on Earth. Although international efforts such as the Montreal Agreement have curbed ozone depletion, the persistence of CFCS means slow but stable recovery. Continuous monitoring and compliance with global policies are essential to restore and protect the ozone layer of future generations.
Source: https://dinhtienhoang.edu.vn
Category: Optical Illusion